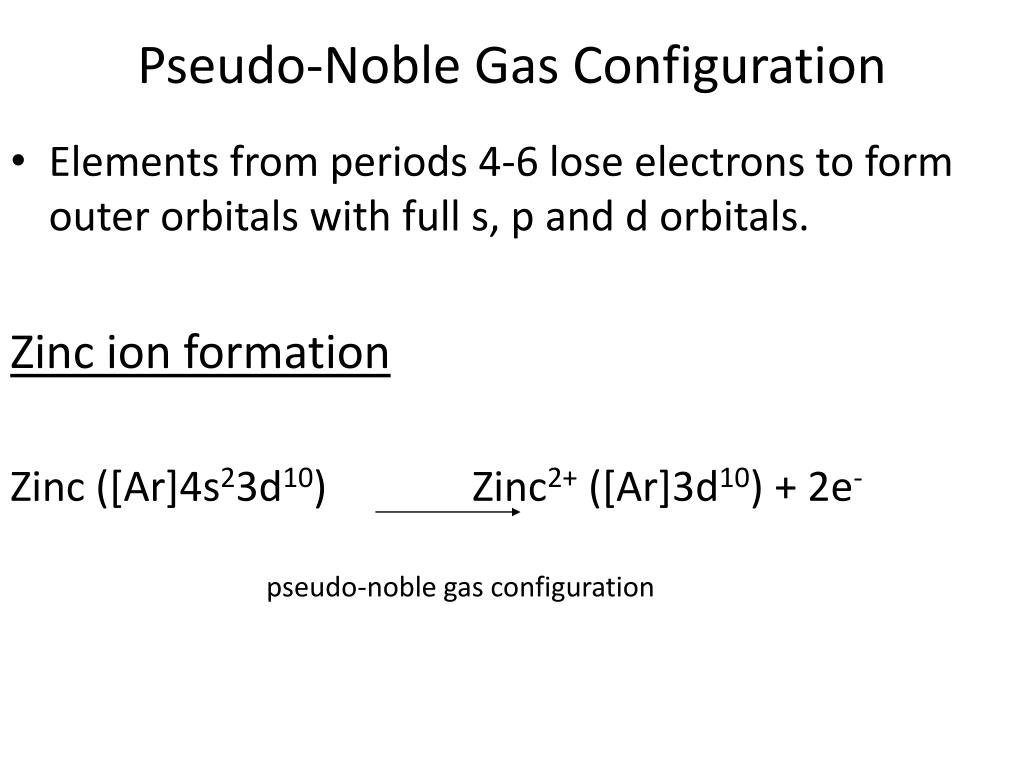
Elements that attain this electronconfiguration. The pseudo noble-gas electron configuration has the outer threeorbitals filled the s p and d- s2p6d10 18 electrons total andso is fairly stable.

Which Of The Following Group 13 Elements Have Pseudo Noble Gas Configuration Is Youtube
Which of the following is a pseudo-noble-gas electron configuration.

. A pseudo-noble-gas configuration has the form s2 p6 d10. A pseudo-noble-gas configuration has the form s2 p6 d10. __________ D a 13.
Hg Xe6s14f145d10 Cu Ar3d10 2. Elements having filled d orbital along with s and p orbitals are said be elements having pseudo inert configuration. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6.
It has 18 electrons in the outer energy level and is a relatively stable configuration. Fe3 Ar3d5 Cd2 Kr4d10 3. Examples of ions with this configuration are Ag Cu Cd2 and Hg2.
The Noble gas electron configuration or the condensed electron configuration for F is He 2s2 3p5. Which of the following ions have pseudo Noble gas configuration Ag K Mg2 Zn2 S2- Sn2 Ga3 O2- N3- cl-. School Southside High School.
Which one of the following electron configurations is considered a pseudo-Nobel gas configuration. Examples of ions with this configuration are Ag Cu Cd2 and Hg2. This is the noble gas core for iodine so the shorthand notation for its electron configuration becomes.
Examples are ions of elements this is because they lose their valence electrons to be fully filled in order to have a stable octet. Which of the following ions has a pseudo-noble-gas electron configuration. The noble gas prior to iodine on the periodic table is krypton Kr which has the electron configuration.
Pseudo-noble gas configuration which can also be called pseudo inert configuration is when elements have fully filled d-orbital along with s - and p - orbitals. Course Title SCIENCE AP. A 1s2 2s2 2p6 3s2 3d10 3f14 B 1s2 2s2 2p6 3s2 3d5 C 1s2 2s2 2p6 3s2 3d6 D 1s2 2s2 2p6 3s2 3d10 E 1s2 2s2 2p6 3s2 3d10 4s2.
How many electrons does silver need to lose in order to achieve a pseudo noble-gas electron. Cu has this electron configuration. Which of the following ions has a pseudo-noble-gas electron configuration.
Up to 24 cash back Pseudo-Noble Gas Configurations Honors Chemistry Write the electron configuration for each ion and then circle the one that is pseudo-stable. Sanskriti nayak 3 years ago. 1s 2s 2p 3s 3p 3d b.
1s2 2s2 2p6 3s2 3p6 3d10. Noble gases have extremely low reactivity because of the fact that the outer shell of electrons for these atoms is completely filled. How many electrons does silver have to give up in order to achieve a pseudo noble gas electron configuration.
K date chemistry name d a 13 which of the following. Which of the following is a pseudo-noble-gas electron configuration. Ar3d10 is the pseudo noble gas configuration Pseudo noble gas configuration meansthere View the full answer Transcribed image text.
1s 2s 2p 3s 3d c. Cadmium has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 commonly written in the shorthand as Kr 5s2. The answer is C Explanation.
1s 2s 2p 3s 3p d. Which of the following is a pseudo noble gas electron configuration a 1 s 2 s 2 from CHE 2022 at Arab Open University Saudi Arabia Branch Study Resources Main Menu. This is called pseudo inert configuration because these elements are having 18 electrons in their valence shell instead of having 8 electrons inert gas configuration.
This preview shows page 1 - 2 out of 2 pages. It has 18 electrons in the outer energy level and is a relatively stable configuration. What is the formula of the ion formed when cadmium achieves a pseudo-noble-gas electron configuration.
AnswerA pseudo-noble gas configuration is. Therefore it is said to have pseudo-noble gas configuration. 1s 2s 2p 3s 3d 4s.
However these non-metallic gases from the periodic table have very low melting and boiling points which makes them useful as coolants in refrigerators.

Ionic And Metallic Bonding Chapter 7 Introduction Ions Are Atoms That Have Either A Positive Or Negative Charge Ions Form To Obtain A More Stable Configuration Ppt Download

Pseudo Noble Gas Electron Configurations Youtube
Ppt Chemical Bonding Powerpoint Presentation Free Download Id 3470834
0 Comments